Selective treatment of intracellular bacterial infections using host cell-targeted bioorthogonal nanozymes†
Abstract
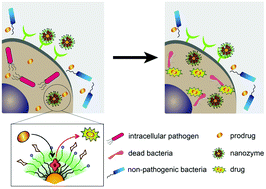
Intracellular bacterial infections are difficult to treat, and in the case of Salmonella and related infections, can be life threatening. Antibiotic treatments for intracellular infections face challenges including cell penetration and intracellular degradation that both reduce antibiotic efficacy. Even when treatable, the increased dose of antibiotics required to counter infections can strongly impact the microbiome, compromising the native roles of beneficial non-pathogenic species. Bioorthogonal catalysis provides a new tool to combat intracellular infections. Catalysts embedded in the monolayers of gold nanoparticles (nanozymes) bioorthogonally convert inert antibiotic prodrugs (pro-antibiotics) into active species within resident macrophages. Targeted nanozyme delivery to macrophages was achieved through mannose conjugation and subsequent uptake VIA the mannose receptor (CD206). These nanozymes efficiently converted pro-ciprofloxacin to ciprofloxacin inside the macrophages, selectively killing pathogenic Salmonella enterica subsp. enterica serovar Typhimurium relative to non-pathogenic Lactobacillus sp. in a transwell co-culture model. Overall, this targeted bioorthogonal nanozyme strategy presents an effective treatment for intracellular infections, including typhoid and tuberculosis.
- This article is part of the themed collections: Materials Horizons 10th anniversary regional spotlight collection: The Americas and Horizons Community Board Collection: Antimicrobial materials and surfaces


 Please wait while we load your content...
Please wait while we load your content...