Remote C(sp3)–H heteroarylation of N-fluorocarboxamides with quinoxalin-2(1H)-ones under visible-light-induced photocatalyst-free conditions†‡
Abstract
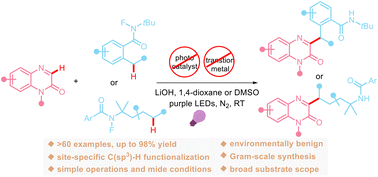
We report, for the first time, a visible-light-promoted remote benzylic/aliphatic C(sp3)–H heteroarylation of N-fluorocarboxamides with quinoxalin-2(1H)-ones. This transformation involves a sequence of amidyl radical generation, 1,5-hydrogen atom transfer (HAT), and cross-dehydrogenative coupling steps. By applying this new method, a broad range of biologically important 3-alkylated quinoxalin-2(1H)-ones (>60 examples) can be readily prepared at room temperature, thus showing the wide utility of this protocol. Notably, this practical reaction occurs under metal- and external photocatalyst-free conditions and exhibits a broad substrate scope, good functional group tolerance, and high chemoselectivity and regioselectivity.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...