‘In-water’, nickel-catalyzed mild preparation of allylic amines employing alcohols: application to ‘all-water’ synthesis of pharmaceuticals†
Abstract
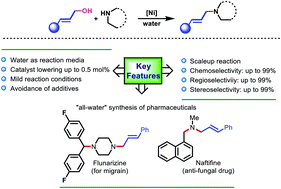
Reported here is a nanomicelle-enabled, ‘in-water’, nickel-catalyzed allylic amination reaction using allylic alcohols under mild conditions (in the absence of additives/bases/activators). Different amines including electron-deficient N-heterocycles and allylic alcohols were found compatible with excellent selectivity (chemo/regio/stereo) and functional group tolerance. Additional features include scale-up synthesis, recycling, reuse of aqueous micelles (up to 5 runs), associated low E-factor, and low residual nickel in the product. Following the developed protocol, an “all-water” synthesis of flunarizine, cinnarizine, and naftifine was reported, wherein the step reactions are promoted by aqueous micelles, leading to a novel water-assisted total synthesis of marketed pharmaceuticals.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...