Stepwise benzylic oxygenation via uranyl-photocatalysis†
Abstract
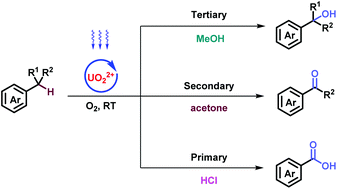
Stepwise oxygenation at the benzylic position (1°, 2°, 3°) of aromatic molecules was comprehensively established under ambient conditions via uranyl photocatalysis to produce carboxylic acids, ketones, and alcohols, respectively. The accuracy of the stepwise oxygenation was ensured by the tunability of catalytic activity in uranyl photocatalysis, which was adjusted by solvents and additives demonstrated through Stern–Volmer analysis. Hydrogen atom transfer between the benzylic position and the uranyl catalyst facilitated oxygenation, further confirmed by kinetic studies. Considerably improved efficiency of flow operation demonstrated the potential for industrial synthetic application.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...