Experimental and quantum chemical study on the transformation behavior of bisphenol S by radical-driven persulfate oxidation†
Abstract
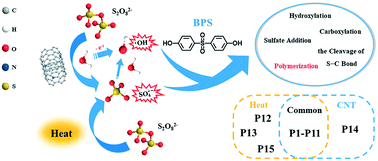
An in-depth study of the degradation of bisphenol S (BPS) by both single-walled carbon nanotubes and heat activated persulfate (PS) was conducted in detail. The effects of various factors, namely, material dosage, initial substrate concentration, initial pH and the water matrix, on the removal of BPS were evaluated, and 10 μM BPS was completely removed in 90 min under the optimal conditions of [BPS]0 : [PS]0 = 1 : 100, T = 25 °C, pH0 = 7.0, and [N-SWCNTs] = 20 mg L−1. Fast removal of BPS was also obtained when the reaction temperature reached 65 °C without catalyst. There were 15 intermediates identified in total, and hydroxylation, sulfate addition, carboxylation, S–C bond cleavage and polymerization were considered to be the main transformation pathways of BPS in both the systems based on LC-MS analysis. The discrepancy in the proportion of hydroxyl and sulfate radicals involved in the two systems led to different distribution and abundance of the observed products. According to quantum chemical calculations, hydroxylation, hydrogen atom abstraction and sulfate addition occurred as the initial reactions between radicals and BPS. Furthermore, the intrinsic reaction coordinate (IRC) paths of the generated primary products were obtained using the program Gaussian 09. Low reaction barriers (22.20, 25.06 and 13.85 kJ mol−1, respectively) revealed that the H atom linked to the phenoxy group and the ortho-C of BPS were the most likely sites to react. The present work reveals the overall transformation behavior of BPS in a radical-triggered PS system by combining experimental and theoretical study.
- This article is part of the themed collection: Best Papers 2022 – Environmental Science: Water Research & Technology


 Please wait while we load your content...
Please wait while we load your content...