Understanding trends in the mercury oxidation activity of single-atom catalysts†
Abstract
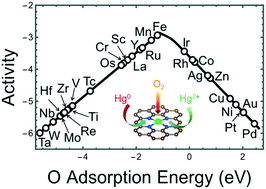
Mercury pollutants emitted from coal-fired power plants are recognized as a global environmental problem. Rapid and sustainable catalytic oxidation of elemental mercury (Hg0) to oxidized mercury (Hg2+) is an essential step to remove mercury from coal-fired power plants. Very recent proof-of-concept experiments firstly found that single-atom catalysts (SACs) have an outstanding performance for Hg0 oxidation. However, looking for an effective catalyst for this reaction is a remaining big challenge. In this work, ten 3d transition metal (TM) SACs with nitrogen-doped carbon substrates (TM1–N4–C, where TM is from Ti to Zn) were designed and analyzed as the catalysts to oxidize Hg0 using O2 as the oxidant. The reaction kinetics and thermodynamics were analyzed based on spin-polarized density functional theory calculations with van der Waals corrections (DFT-D3). We found that Fe1–N4–C has the highest catalytic activity with the lowest energy barrier in the rate-determining step. By analyzing the relationships between reaction thermodynamics and kinetics, the adsorption energy of atomic O was found as an effective descriptor that can predict the rate-determining step barriers of 72 catalysts. The predicted values of the energy barriers were then successfully verified by subsequent DFT-D3 calculations. To further understand the trends in Hg0 oxidation, a volcano-shaped microkinetic model was derived based on the linear scaling relations of the reaction kinetics and thermodynamics, as a function of O adsorption energy. The catalytic activities of 28 transition metal SACs were then predicted by the volcano map, showing that Fe1–N4–C has the highest catalytic activity among the analyzed 3d, 4d, and 5d SACs. Furthermore, correlations between activity and electronic structure were discussed through the analyses with Bader charge, d-band center, and system electronegativity. Most importantly, this study provides an understanding of the activity trends of Hg0 oxidation and a descriptor-based design guideline for this environmentally important reaction.


 Please wait while we load your content...
Please wait while we load your content...