Prediction of nanomagnetite stoichiometry (Fe(ii)/Fe(iii)) under contrasting pH and redox conditions†
Abstract
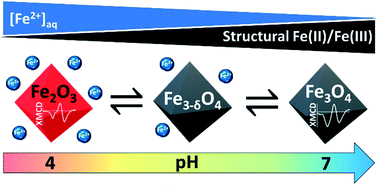
Magnetite (Fe(III)2Fe(II)O4) nanoparticles are fascinating nanoparticulate minerals due to their electronic, magnetic and chemical properties. Ubiquitous in the environment, they are also among the most used ferromagnetic nanomaterials in environmental, industrial and biomedical applications. Their intriguing structural and reactivity features do not only arise from the “nano-effect” but also from the occurrence of Fe2+ ions in their structure. Previous studies showed that partial oxidation of (nano)magnetite may occur. However, such transformations were only monitored under either oxidizing or very acidic conditions. Here, we report that 10 nm-sized stoichiometric magnetite particles (Fe(II)/Fe(III) = 0.5) are in fact not stable in aqueous solutions over a biologically and environmentally relevant pH range (4–7). In the absence of O2, an H+-promoted dissolution process is responsible for the preferential release of Fe(II) into solution, which leads to partial oxidation of magnetite to a magnetite–maghemite solid solution. Long-term kinetic investigations combined with XMCD measurements reveal that the dynamic exchange of Fe(II) between the surface and the solution is key to determining the magnetite stoichiometry even at circumneutral pH. Based on this finding, we developed a thermodynamic model for the magnetite–maghemite solid solution able to predict the chemical stability of the 10 nm-sized magnetite. This model enables the behavior and transformation of magnetite nanoparticles in aqueous solutions to be rationalized and predicted, which is crucial for a broad range of applications (medicine, biology, chemistry, environment, etc.).
- This article is part of the themed collections: Environmental fate of nanomaterials and Environmental Science: Nano Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...