The effects of metal cofactors on the reactivity of quercetin 2,4-dioxygenase: synthetic model studies with M(ii)-complexes (M = Mn, Co, Ni, Cu, Zn) and assessment of the regulatory factors in catalytic efficacy†‡
Abstract
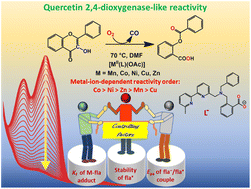
This paper demonstrates the metal ion effects on the quercetin 2,4-dioxygenase (2,4-QD)-like reactivity. For this purpose, a series of five metal(II)–acetato complexes [MII(L)(OAc)] {M = Mn (1OAc), Co (2OAc), Ni (3OAc), Cu (4OAc), Zn (5OAc); OAc = acetate} supported with a newly designed N3O-donor carboxylato ligand L− {L− = 2-((benzyl((6′-methyl-[2,2′-bipyridin]-6-yl)methyl)amino)methyl)benzoate} has been synthesised as models for the active sites of MII-substituted 2,4-QDs. The enzyme–substrate (ES) model complexes [MII(L)(fla)] {M = Mn (1fla), Co (2fla), Ni (3fla), Cu (4fla), Zn (5fla); flaH = flavonol} have been synthesised by reacting flaH with their corresponding acetate-bound complexes in basic conditions. Detailed physicochemical properties of all the compounds are reported. Furthermore, single-crystal X-ray diffractions have been done to determine the structures of the compounds 2OAc·2H2O, 3OAc, 4OAc·CH2Cl2·2H2O, 5OAc·2H2O and 2fla·MeOH. The enzymatic reactivities of complexes 1OAc–5OAc towards the dioxygenation of flavonol have been explored in detail. All the complexes effectively catalyse the oxygenative degradation of flavonol in N,N-dimethylformamide (DMF) medium at 70 °C under multiple-turnover conditions and produce enzyme-type products. Kinetic investigations were performed to see the metal ions’ effects on reactivity. The reaction rates vary with the metal ions, showing the order Co > Ni > Zn > Mn > Cu. The studies reveal that the reactivities of the [MII(L)(OAc)] complexes are governed primarily by three factors viz the ES adduct formation constant (Kf), the redox potential (Epa) of the bound fla−/fla˙ couple, and the degree of delocalisation of the fla˙ radical with the metal electrons, which are drastically influenced by the M2+ ions. In the mechanistic interpretation, a single-electron transfer (SET) from the bound-flavonolate to dioxygen has been proposed to generate the catalytically important “M(II)–fla˙” radical and superoxide ion, which react further to bring about the dioxygenation reaction. The identification of the metal(II)-bound flavonoxy radical intermediate for the case of cobalt using EPR spectroscopy and the detection of superoxide ion by NBT2+ test and EPR spin-trapping experiment (DMPO test) are remarkable in envisaging the reaction pathway.


 Please wait while we load your content...
Please wait while we load your content...