All-inorganic ferric wheel based on hexaniobate-anion linkers†
Abstract
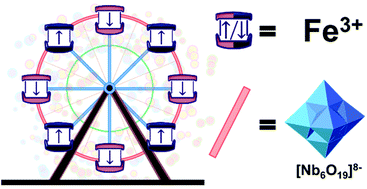
Utilizing the inherent ability of Lindquist-type hexaniobate cluster-anions, [Nb6O19]8−, to serve as oxo-donor ligands in complexes with transition-metal cations, we report the synthesis and characterization of the first all-inorganic “ferric” wheel, Li48[(Nb6O19)8Fe8(OH)8]·88H2O, comprised of eight Fe atoms linked by eight hexaniobate cluster-anion ligands. Bond valence sum analysis of the X-ray structure and the synthesis conditions themselves indicate that the Fe atoms are in the +3 oxidation state. This is confirmed by magnetic susceptibility and electron paramagnetic resonance (EPR) measurements which indicate the presence of high spin (S = 5/2) Fe(III) ions. In addition, magnetic susceptibility measurements reveal long-range superexchange antiferromagnetic interactions between the hexaniobate-ligand separated Fe3+ ions (J = −0.22 cm−1). More generally, the results suggest the use of hexaniobate cluster-anions as linkers in the synthesis of other two- or three-dimensional polyoxometalate framework structures.


 Please wait while we load your content...
Please wait while we load your content...