Lewis acid–base adducts of Al(N(C6F5)2)3 and Ga(N(C6F5)2)3 – structural features and dissociation enthalpies†
Abstract
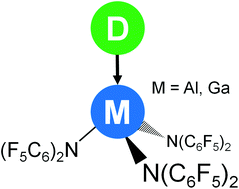
Herein we present the molecular structures of six neutral Lewis acid–base adducts of the Lewis superacid Al(N(C6F5)2)3 and its higher homolog Ga(N(C6F5)2)3 with the electron pair donors MeCN, CNtBu, THF and PMe3. The crystal structures reveal crucial structural changes compared to the free Lewis acids as a consequence of the adduct formation. Furthermore, we calculated the corresponding dissociation enthalpies of the adducts which lie between 69 and 141 kJ mol−1 and are therefore considerably lower compared to the values for the formation of the anionic fluoride or chloride metallates.
- This article is part of the themed collection: Spotlight Collection: Fluorinated ligands


 Please wait while we load your content...
Please wait while we load your content...