Hetero- and homoleptic binuclear gold(i)–thiolate and –halide complexes – ligand exchange kinetics and supramolecular structures†
Abstract
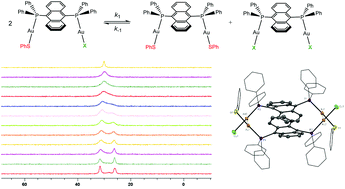
Heteroleptic and homoleptic binuclear Au(I) complexes [Au2(μ-PAnP)(SPh)(X)] (X = Cl− or Br−), [Au2(μ-PAnP)(SPh)2] and [Au2(μ-PAnP)(SPhCO2H)2] (SPh = benzenethiolate and SPhCO2H = 4-thiolatobenzoic acid) containing the bridging diphosphine, 9,10-bis(diphenylphosphino)anthracene (PAnP), were synthesized and characterized by single crystal X-ray diffraction. [Au2(μ-PAnP)(SPh)2] exists as a monomer in its crystals but [Au2(μ-PAnP)(SPhCO2H)2] polymerizes into zig-zag chains via intermolecular hydrogen bonding. [Au2(μ-PAnP)(SPh)(Cl)] forms cyclophane-like dimers of Ci symmetry in crystals via intermolecular aurophilic interactions (Au–Au distance = 3.3081(5) Å). Recrystallization of [Au2(μ-PAnP)(SPh)(Br)] invariably led to crystals composed of [Au2(μ-PAnP)(SPh)(Br)] and [Au2(μ-PAnP)(Br)2]. Despite the chemically different P atoms in the heteroleptic [Au2(μ-PAnP)(SPh)(Cl)] and [Au2(μ-PAnP)(SPh)(Br)], solutions of the complexes show only a single signal in their 31P{1H} NMR spectra at room temperature which resolved into two singlets of equal intensity at 183 K. Identical signals which show the same thermal behavior were observed in solutions of [Au2(μ-PAnP)(SPh)2] and [Au2(μ-PAnP)(X)2] in 1 : 1 molar ratios, indicating that there are three exchanging species, [Au2(μ-PAnP)(SPh)(X)], [Au2(μ-PAnP)(SPh)2] and [Au2(μ-PAnP)(X)2], in solution. A solution of [Au2(μ-PAnP)(Cl)2] and [Au2(μ-PAnP)(Br)2] in 1 : 1 molar ratio shows two singlets, implying that the exchange is not due to the dissociation of either PAnP or halide ligands, but rather it involves the exchange of the thiolate and the halide ligands (SPh− ↔ X−). A mixture of [(PPh3)Au(SPh)] and [(PPh3)Au(Cl)] (1 : 1 molar ratio) showed only one signal in its room temperature 31P{1H} NMR spectrum, indicating that the ligand exchange can happen intermolecularly. Self-exchange of SPh− ligands is possible as the room temperature 31P NMR spectrum of a mixture of [Au2(μ-PAnP)(SPh)2] and [Au2(μ-PAnP)(SPhCO2H)2] displayed only one signal. The rate constants of the exchange were determined by fitting the line shapes of the 31P NMR signals at different temperatures. The activation energies (Eas), obtained from Arrhenius plots, for the SPh− ↔ Cl− and SPh− ↔ Br− exchange are 36.9 ± 0.7 and 33.7 ± 1.0 kJ mol−1, respectively. The activation enthalpy and activation entropy, obtained from Eyring plots, for the SPh− ↔ Cl− and SPh− ↔ Br− exchange are 35.0 ± 0.7 kJ mol−1 and −25.7 ± 3.2 J K−1, and 32.0 ± 1.0 kJ mol−1 and −21.8 ± 4.7 J K−1, respectively. Based on the kinetic results, two possible mechanisms were proposed for the reactions.


 Please wait while we load your content...
Please wait while we load your content...