Base-stabilized formally zero-valent mono and diatomic molecular main-group compounds
Abstract
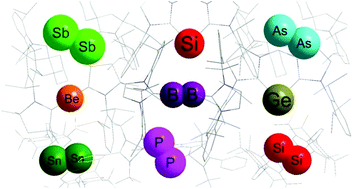
Various compounds are known for transition metals in their formal zero-oxidation state, while similar compounds of main-group elements are recently realized and limited to only a few examples. Lewis-base-stabilized mono and diatomic molecular species (B2, C, C2, Si, Si2, Ge, Ge2, Sn, P2, As2, Sb2) represent groundbreaking examples of main-group compounds with formally zero-oxidation state. In recent years, the isolation of low-valent main-group compounds has attracted increasing attention of both experimental and theoretical chemists. This is not only due to their fascinating electronic structures and exceptional reactivities, but also their use as valuable precursors for the synthesis of exotic yet important chemical species. This has led to a better understanding of the intricate balance of the donor–acceptor properties of the ligand(s) used to stabilize elements in a formally zero-oxidation state. Owing to the unusual oxidation state of the central element, many compounds containing formally zero-valent elements can efficiently activate otherwise inert small molecules. This review describes the synthesis, characterization, and reactivity of reported mono and diatomic formal zero-oxidation state main-group compounds. This review also emphasizes the comparative description of systems where different ligands are used to stabilize an element in its formal zero-oxidation state.
- This article is part of the themed collection: 2022 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...