Structural diversity and hydrogen storage properties in the system K–Si–H†
Abstract
KSiH3 exhibits 4.1 wt% experimental hydrogen storage capacity and shows reversibility under moderate conditions, which provides fresh impetus to the search for other complex hydrides in the K–Si–H system. Here, we reproduce the stable Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase of K2SiH6 and uncover two denser phases, space groups P
m phase of K2SiH6 and uncover two denser phases, space groups P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1 and P63mc at ambient pressure, by means of first-principles structure searches. We note that P
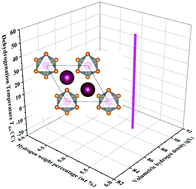
m1 and P63mc at ambient pressure, by means of first-principles structure searches. We note that P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1-K2SiH6 has a high hydrogen content of 5.4 wt% and a volumetric density of 88.3 g L−1. Further calculations suggest a favorable dehydrogenation temperature Tdes of −20.1/55.8 °C with decomposition into KSi + K + H2. The higher hydrogen density and appropriate dehydrogenation temperature indicate that K2SiH6 is a promising hydrogen storage material, and our results provide helpful and clear guidance for further experimental studies. We found three further potential hydrogen storage materials stable at high pressure: K2SiH8, KSiH7 and KSiH8. These results suggest the need for further investigations into hydrogen storage materials among such ternary hydrides at high pressure.
m1-K2SiH6 has a high hydrogen content of 5.4 wt% and a volumetric density of 88.3 g L−1. Further calculations suggest a favorable dehydrogenation temperature Tdes of −20.1/55.8 °C with decomposition into KSi + K + H2. The higher hydrogen density and appropriate dehydrogenation temperature indicate that K2SiH6 is a promising hydrogen storage material, and our results provide helpful and clear guidance for further experimental studies. We found three further potential hydrogen storage materials stable at high pressure: K2SiH8, KSiH7 and KSiH8. These results suggest the need for further investigations into hydrogen storage materials among such ternary hydrides at high pressure.


 Please wait while we load your content...
Please wait while we load your content...