Highly stable actinide(iii) complexes supported by doubly aromatic ligands†
Abstract
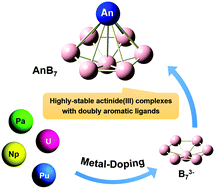
Owing to the electron-deficient nature of boron atoms, the structures and properties of boron clusters can be enriched by doping various metal atoms, including lanthanide metal atoms. Nevertheless, the viability of actinide analogues has not been fully elucidated up to now. Here we demonstrate a series of highly stable low-valent actinide(III) boron clusters AnB7 (An = Pa, U, Np, and Pu) using first-principles calculations. The predicted global minimum structures of all the AnB7 complexes possess half-sandwich geometries with C6v symmetry and belong to MIII[B7]3−-type species. In each AnB7 species, the B73− ligand possesses double aromaticity features with six delocalized π electrons and six delocalized σ electrons. Bonding analysis shows that although there is a substantial contribution of electrostatic interaction in each cluster, covalent interaction is responsible for the stability of AnB7. All the AnB7 species show significantly high formation energies, especially for NpB7, which is in line with the stronger Np-B covalent bonds. In addition, the simulated photoelectron spectroscopy analysis confirms the high electronic stability of neutral AnB7. These results imply that these ultrastable actinide(III) complexes are accessible in the gas phase at room temperature. This work may provide a theoretical basis for the design of highly stable boron-based nanomaterials as well as preparation of low-valent actinide complexes.


 Please wait while we load your content...
Please wait while we load your content...