Synthesis and characterization of a bimetallic americium(iii) pyrithionate coordination complex†
Abstract
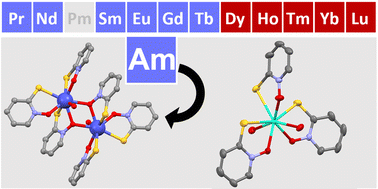
The aqueous reaction of sodium pyrithione, (Na)mpo, with 243AmCl3·nH2O yields a dimerized complex, [243Am(mpo)2(μ-O-mpo)(H2O)]2·3H2O. This compound is compared with isostructural lanthanide pyrithionates, where dimerization across the 4f-block is observed to be dependent upon the size of the cation. Unlike in most reported Am(III) UV-visible absorption spectra, [243Am(mpo)2(μ-O-mpo)(H2O)]2·3H2O shows significant splitting in the fingerprint excitations. This is attributed to a unique ligand-field environment, where the Am–mpo bonds possess different bonding compared to the Nd(III) analog because of increasing covalent interactions.
- This article is part of the themed collection: Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...