Nickel-catalyzed decarboxylative cross-coupling of indole-3-acetic acids with aryl bromides by convergent paired electrolysis†
Abstract
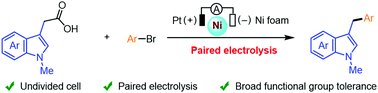
Herein, nickel-catalyzed decarboxylative cross-coupling of indole-3-acetic acids with aryl bromides by convergent paired electrolysis was developed in an undivided cell. This protocol features good functional group tolerance, and is chemical redox agent- and sacrificial electrode-free. Mechanistic studies indicated that the base was crucial for the decarboxylation step and a NiI/NiIII catalytic cycle was involved in this transformation.
- This article is part of the themed collections: 2022 Pioneering Investigators and Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...