Redox- and metal-directed structural diversification in designed metalloprotein assemblies†
Abstract
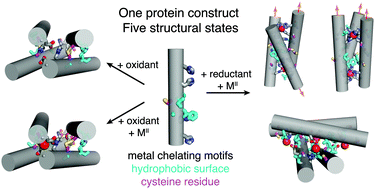
Herein we describe a designed protein building block whose self-assembly behaviour is dually gated by the redox state of disulphide bonds and the identity of exogenous metal ions. This protein construct is shown – through extensive structural and biophysical characterization – to access five distinct oligomeric states, exemplifying how the complex interplay between hydrophobic, metal–ligand, and reversible covalent interactions could be harnessed to obtain multiple, responsive protein architectures from a single building block.


 Please wait while we load your content...
Please wait while we load your content...