Ring-opening of a thorium cyclopropenyl complex generates a transient thorium-bound carbene†
Abstract
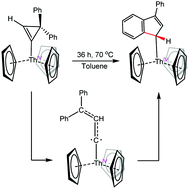
The reaction of [Cp3ThCl] with in situ generated 1-lithium-3,3-diphenylcyclopropene results in the formation of [Cp3Th(3,3-diphenylcyclopropenyl)] (1), in good yields. Thermolysis of 1 results in isomerization to the ring-opened product, [Cp3Th(3-phenyl-1H-inden-1-yl)] (3) via a hypothesized carbene intermediate. This transformation represents a new mode of reactivity of 3,3-diphenylcyclopropene with the actinides, improving our ability to use this reagent as a carbene source. A combined DFT and 13C{1H} NMR analysis of 1 shows a spin–orbit induced downfield shift at Cα due to participation of the 5f orbitals in the Th–C bond.
- This article is part of the themed collection: Celebrating 10 years of ChemComm Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...