Ethylene oxide functionalization enhances the ionic conductivity of a MOF†
Abstract
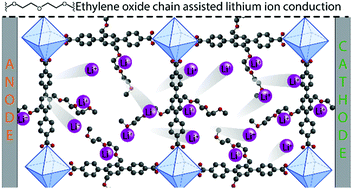
Varying the degree of ethylene oxide (EO) functionalization of the zirconium MOF UiO-68 affords two novel MOFs; UiO-68-EO and UiO-68-2EO exhibit solvent-free ionic conductivity upon loading LiTFSI in their pores. Incorporating EO chains provides a pathway for lithium ion migration between the coordinated sites and results in an ionic conductivity of 3.8 × 10−7 S cm−1 and 3.9 × 10−4 S cm−1 at 90 °C for UiO-68-EO/LiTFSI and UiO-68-2EO/LiTFSI respectively.
- This article is part of the themed collection: Emerging Trends in MOFs


 Please wait while we load your content...
Please wait while we load your content...
