Migratory insertion of copper-allenylidene from propargyl ester†
Abstract
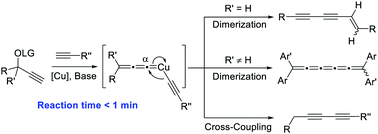
The highly efficient copper-catalyzed homo-dimerization and cross-coupling of propargyl esters have been developed. Various 1-en-3,5-diynes, [5]cumulenes and 1,3-diynes were successfully furnished via the copper-allenylidene intermediates with moderate to excellent yields. Migratory insertion is proposed as the key step to achieve the selectivity at the carbene carbon of the copper-allenylidene.
- This article is part of the themed collections: 2022 Pioneering Investigators and Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...