Azidoborolate anions and azidoborole adducts: isolable forms of an unstable borole azide†
Abstract
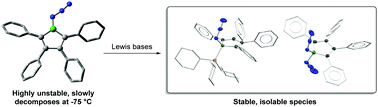
Boroles are well known to undergo ring expansion reactions with organic azides to yield 1,2-azaborinines. A synthon featuring both azide and borole moieties within the same molecule, 1-azido-2,3,4,5-tetraphenylborole, was found to be much less stable than the related, previously-reported azidoborafluorene and decomposed to intractable mixtures well below room temperature. It could, however, be trapped at −75 °C through the formation of Lewis base adducts, even in the form of the “azide-stabilized azidoborole” complex anion diazidoborolate. DFT calculations provide a rationale for the low stability of the azidoborole under study.
- This article is part of the themed collection: Boron Chemistry in the 21st Century: From Synthetic Curiosities to Functional Molecules


 Please wait while we load your content...
Please wait while we load your content...