Tuning of pH enables carbon-13 hyperpolarization of oxalates by SABRE†
Abstract
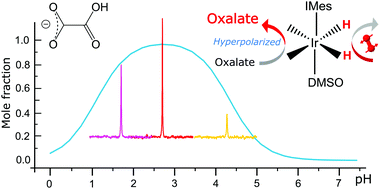
Nuclear spin hyperpolarization transforms typically weak NMR responses into strong signals paving the way for low-gamma nuclei detection within practical time-frames. SABRE (Signal Amplification by Reversible Exchange) is a particularly popular hyperpolarization technique due to its simplicity but the pool of molecules it can polarize is limited. The recent advancement in the form of co-ligands has made SABRE applicable towards molecules with O-donor sites e.g. pyruvate, a key step towards its potential clinical application. Here we explore the SABRE hyperpolarization of another compound with an alpha-keto motif, namely oxalate. We show that hyperpolarization of oxalate may be achieved by adjusting the pH in the presence of sulfoxide co-ligands. The SABRE effect for oxalate in methanol solutions is most effective for the mono-protonated form, which is dominant in the solution around pH ∼2.8. The polarization levels become markedly lower at both higher and lower pH. Employing 50% enriched pH2 we achieve up to 0.33% net 13C polarization in mono-protonated oxalate. In an alternative procedure we show that the hyperpolarization effect in oxalates can also be realised by synthesizing an esterified version of it, without any substantive pH implications. Further, the procedures to create hyperpolarized singlet orders in such substrates are also investigated.
- This article is part of the themed collection: Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...