Unnatural cyclodextrins can be accessed from enzyme-mediated dynamic combinatorial libraries†
Abstract
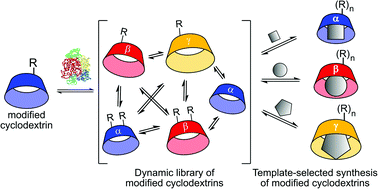
Dynamic systems of cyclodextrins (CDs) enabled by a native cyclodextrin glucanotransferase (CGTase) can incorporate unnatural glucopyranose-derived building blocks, expanding the applicability of enzyme-mediated dynamic combinatorial chemistry by using synthetically modified substrates. Starting dynamic combinatorial libraries from CDs with a single 6-modified glucopyranose results in a dynamic mixture of CDs containing several modified glucopyranoses. The relative concentrations of modified α, β or γ-CDs can be controlled by the addition of templates, providing a novel way to access modified CDs.
- This article is part of the themed collection: Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...