A chemo- and regiocontrolled approach to bipyrazoles and pyridones via the reaction of ethyl 5-acyl-4-pyrone-2-carboxylates with hydrazines†
Abstract
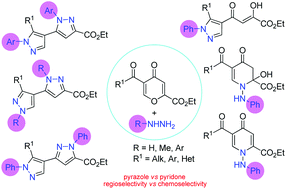
Chemo- and regiocontrolled syntheses of pyrazoles and pyridones are presented on the basis of 4-pyrones. A novel approach towards highly functionalized 3,4′-bipyrazoles has been developed by using reactions of ethyl 5-acylcomanoates with hydrazines. The acid-promoted double cyclocondensation allows switching of the structure of the pyrazole rings easily through changing both the nature of hydrazine and the reaction conditions. The transformation of 4-pyrones with phenylhydrazine in EtOH at −20 °C leads to hydroxypyridones as monoaddition products which can be used as precursors for the preparation of pyridone and pyrazole derivatives. A novel rearrangement of 2-hydroxypyridone to pyrazolyl-1,3-diketones in DMSO was found. The structure and regiochemistry of bipyrazoles were confirmed by X-ray analysis and 2D NMR experiments.


 Please wait while we load your content...
Please wait while we load your content...