Thermal crosslinking of PBI/sulfonated polysulfone based blend membranes
Abstract
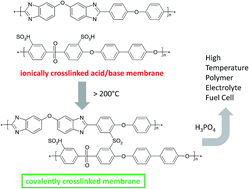
Crosslinked polybenzimidazole (PBI) membranes are most often obtained by reacting the nitrogen atoms of PBI with an alkylating agent. These links can be attacked by nucleophiles at elevated temperatures. To avoid N–CH2-links we introduce a new method to crosslink PBI, starting from ionically crosslinked acid/base blend membranes. By heating them to temperatures above say 200 °C, a Friedel–Crafts reaction between sulfonic acid groups and electron rich phenyl groups covalently crosslinks the acid and base components in the blend by chemically stable aromatic sulfone bonds. According to the literature pure PBI can also be cured and a radical mechanism involving air was suggested. We show that PBI can also be cured in an inert atmosphere. We propose that the thermal curing of pure PBI, which necessitates slightly higher temperatures than blend membranes, proceeds via hydrolysis of imidazole to –COOH and diamine, followed by a Friedel–Crafts reaction of the acid. While crosslinks cannot be directly analysed by nmr or IR, our data support the mentioned mechanism. We show the effect of curing temperature and time on membrane properties like solubility, phosphoric acid uptake and mechanical properties, and test a membrane in a fuel cell, proving that the membranes are gas tight and show a good performance.

 Please wait while we load your content...
Please wait while we load your content...