Electronic interaction between transition metal single-atoms and anatase TiO2 boosts CO2 photoreduction with H2O†
Abstract
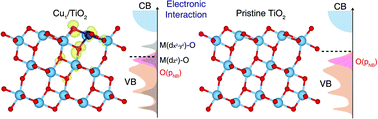
Single-atom catalysts are playing a pivotal-role in understanding atomic-level photocatalytic processes. However, single-atoms are typically non-uniformly distributed on photocatalyst surfaces, hindering the systematic investigation of structure–property correlation at atomic precision. Herein, by combining material design, spectroscopic analyses, and theoretical studies, we investigate the atomic-level CO2 photoreduction process on TiO2 photocatalysts with uniformly stabilized transition metal single-atoms. First, the electronic interaction between single Cu atoms and the surrounding TiO2 affects the reducibility of the TiO2 surface, leading to spontaneous O vacancy formation near Cu atoms. The coexistence of Cu atoms and O vacancies cooperatively stabilizes CO2 intermediates on the TiO2 surface. Second, our approach allows us to control the spatial distribution of uniform single Cu atoms on TiO2, and demonstrate that neighboring Cu atoms simultaneously engage in the interaction with CO2 intermediates by controlling the charge localization. Optimized Cu1/TiO2 photocatalysts exhibit 66-fold enhancement in CO2 photoreduction performance compared to the pristine TiO2.


 Please wait while we load your content...
Please wait while we load your content...