Study on the kinetics of the adsorption and desorption of NH3 on Fe/HBEA zeolite
Abstract
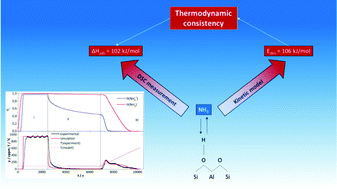
In this work, a Fe/HBEA zeolite (Si/Al: 12.5), representing an effective catalyst for the NH3-SCR process, was physico-chemically characterized and investigated regarding the kinetics of the adsorption and desorption of NH3. The sample was evaluated by N2 physisorption, 57Fe Möessbauer and DRUV-Vis spectroscopy, while the kinetics was investigated by temperature-programmed desorption of NH3 (TPD) including different adsorption temperatures. It was shown that the NH3 chemisorption results in weakly and strongly bonded molecular ammonia as well as ammonium species. A kinetic mean field model was developed implying two different types of adsorbates reflecting low (<ca. 200 °C) and high temperature desorption of NH3 (>ca. 200 °C). Kinetic parameters and surface coverages were obtained from numeric fits of the TPD curves, whereas pre-exponential factors of adsorption were deduced from the kinetic gas theory. As a result, the activation energy for the NHx adsorbate decomposition in the low temperature regime, which is assigned to single and double bonded ammonium species was determined to be 106 kJ mol−1. The NH3 desorption at higher temperatures referred to an activation energy of 133 kJ mol−1 predominately related to NH3 coordinated to Lewis acid surface sites and to some extent to stabilized NH4+ species. For validation of the kinetic model, experiments were simulated including NH3 adsorption at different temperatures, subsequent flushing with N2 and final TPD. Additionally, the consistency of the activation energies with the thermodynamic data was checked using differential scanning calorimetry and a van’t Hoff approach.


 Please wait while we load your content...
Please wait while we load your content...