An amino-type halogen-bonded organic framework for the selective adsorption of aliphatic acid vapors: insight into the competitive interactions of halogen bonds and hydrogen bonds†
Abstract
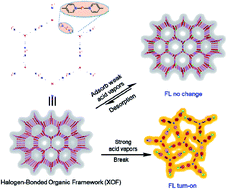
As new members of the organic framework family, halogen-bonded organic frameworks (XOFs) have the potential to exhibit gas capture and vaporchromic behavior, yet they remain to be explored. Herein, the novel amino type of 2D XOF-TPPA was successfully constructed via [N⋯I+···N] interactions between a pyridyl-modified triphenylamine ligand (TPPA) and iodonium ions. The formation of XOF-TPPA was sufficiently characterized via X-ray photoelectron spectroscopy (XPS), 1H NMR, IR, TEM, HRTEM, PXRD, selected area electron diffraction (SAED), and theoretical simulations. Due to differences in electron-donating ability between the pyridyl N1 atom and amino N2 atom, XOF-TPPA serves as an efficient and recyclable adsorbent for MeCOOH/EtCOOH vapors. The absorption capacity of XOF-TPPA for MeCOOH and EtCOOH vapors was 418 and 173 mg g−1, respectively. The absorption properties are largely dependent on the competitive interactions of [N1⋯I+···N1] halogen bonds and [N1/N2⋯HOOC–R] hydrogen bonds. This competitive response mechanism not only brings further understanding of the noncovalent interactions, but it also provides a novel strategy for the design of stimulus-responsive materials.


 Please wait while we load your content...
Please wait while we load your content...