Effects on the photovoltaic properties of copolymers with five-membered chalcogen-π-heterocycle bridges†
Abstract
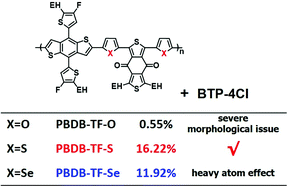
Five-membered aromatic heterocycles are widely used for constructing conjugated polymers in the application of organic photovoltaic cells (OSCs). In this work, three polymers containing furan, thiophene, and selenophene as the π-bridge in their backbones, namely PBDB-TF-O, PBDB-TF-S and PBDB-TF-Se, were designed, synthesized and characterized. By blending with BTP-4Cl, an emerging non-fullerene acceptor, the OSC devices based on the three polymer donors show distinct photovoltaic properties with the variation of the five-membered chalcogen-π-heterocycles. It is demonstrated that PBDB-TF-O tends to undergo severe aggregation while blending with the NFA molecule due to its strong polarity by the introduction of oxygen atoms, and the heavy atom effect of Se exists in PBDB-TF-Se, resulting in its poor luminescence property and larger non-radiative energy loss. The PCE is 0.55% and 11.92% for PBDB-TF-O and PBDB-TF-Se based OSCs, respectively. PBDB-TF-S, the polymer containing thiophene as the heterocycle-π-bridge, presents appropriate aggregation characteristics, decreased non-radiative energy loss, and high and balanced mobilities, and thus presents a desirable PCE of 16.22%. This work indicates that thiophene should be the most appropriate unit among the three chalcogen-heterocycles to construct the backbones of conjugated polymers with high photovoltaic performance.


 Please wait while we load your content...
Please wait while we load your content...