Fluorinated phosphonium ionic liquid boosts the N2-adsorbing ability of TiO2 for efficient photocatalytic NH3 synthesis†
Abstract
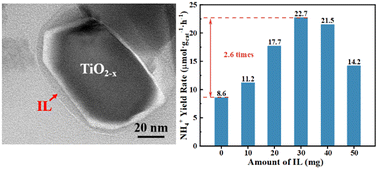
Photocatalytic N2 reduction (pNRR) is considered to be an important pathway for green NH3 synthesis. However, in the traditional photocatalytic system, the adsorption capacity of N2 by an aqueous phase reaction solution and a semiconductor photocatalyst is limited, which seriously restricts the performance improvement of photocatalytic N2 reduction synthesis of NH3. In this work, a TiO2 photocatalyst with a positively charged surface (TiO2−x) was synthesized, and low-cost fluorinated phosphonium ionic liquid (IL) tetrabutylphosphate–hexafluorophosphate ([P4,4,4,4][PF6]) was innovatively added to the water phase reaction system. Notably, the anion [PF6]− of [P4,4,4,4][PF6] can be electrostatically driven to enrich on TiO2−x (IL-TiO2−x), creating an outermost layer that transforms the surface properties from hydrophilic to aerophilic, thus effectively promoting N2 adsorption. Under full solar irradiation, the NH4+ generation rate of IL-TiO2−x substantially increased by 2.6 times (22.7 μmol gcata−1 h−1) compared to the system without IL was rendered. This intriguing work offers a feasible strategy for developing an efficient N2 capture–fixation reaction system for pNRR.


 Please wait while we load your content...
Please wait while we load your content...