Regulating solvation shells and interfacial chemistry in zinc-ion batteries using glutaronitrile based electrolyte†
Abstract
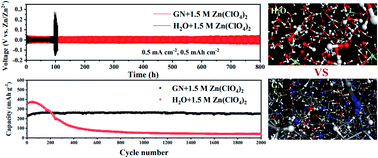
The dendrite growth, parasitic reactions and dissolution of cathodes caused by numerous free H2O molecules in aqueous electrolyte can lead to rapid performance degradation, limiting the application of aqueous Zn-ion batteries (AZIBs). Here, a new glutaronitrile (GN) based electrolyte is introduced by coupling a hydrated Zn salt (Zn(ClO4)2·6H2O) and GN to mitigate these issues. Raman spectroscopy and molecular dynamics simulations demonstrate that the strong coordination between Zn2+ and GN can effectively regulate solvation shells of Zn2+ ions. Moreover, the H2O molecules mostly exist in the confined state rather than in the free state, ensuring the excellent electrochemical performances. Benefiting from this unique solvation shell of Zn2+ ions, Zn‖Zn symmetric cells can operate steadily for more than 800 h without Zn dendrite growth, and Zn‖Cu half-cells achieve extremely reversible zinc plating/stripping with coulombic efficiencies of 99.0%. XPS suggests that the in situ formed ZnO-rich solid electrolyte interface (SEI) in this GN based electrolyte can effectively inhibit the occurrence of parasitic reactions, improving the stability of the battery. Thus, the Zn‖NH4V4O10 full cells exhibit a high specific capacity of 239 mA h g−1 at 5C over 2500 cycles without significant capacity decay. The differences in the electrochemical reaction mechanism of the NH4V4O10 cathode in between ZHO and ZGN have been discussed in detail.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...