Improving the photo-oxidative capability of BiOBr via crystal facet engineering†
Abstract
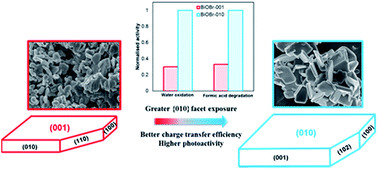
Bismuth oxybromide (BiOBr) has emerged as a potential visible-light-driven photocatalyst with relatively high photocatalytic activity although modifications are still necessary to further promote its photocatalytic performance. Here, a facile chemical precipitation process was used to synthesize tetragonal BiOBr with a predominance of either {001} or {010} exposed crystal facets. Scanning electron microscopy revealed that BiOBr particles dominated by the {010} facet possessed a large plate-like morphology while the {001}-dominated BiOBr comprised smaller, more irregular particles. Ultraviolet-visible diffuse reflectance spectra and Mott–Schottky analysis highlighted a difference in electronic band structure of the two materials; BiOBr-010 possessed a valence band potential and a band-gap of 2.71 and 2.95 eV versus normal hydrogen electrode (NHE), respectively, while BOBr-001 exhibited values of 2.63 and 3.15 eV versus NHE, respectively. BiOBr-010 displayed a better photo-oxidative capability than BOBr-001 for both water oxidation and formic acid degradation (aqueous phase). The higher photo-oxidative capability of BiOBr-010 was attributed to the suppression of photo-induced electron/hole recombination. Additionally, the improved charge transfer efficiency and reduced charge transfer resistance in BiOBr-010 was revealed to be beneficial for enhancing photoelectrochemical (PEC) performance. The findings account for the better photo-oxidative activity and higher current density of BiOBr-010 despite its smaller specific surface area and illustrate the use of crystal facet engineering to promote photocatalytic performance.


 Please wait while we load your content...
Please wait while we load your content...