Decoupled amphoteric water electrolysis and its integration with Mn–Zn battery for flexible utilization of renewables†
Abstract
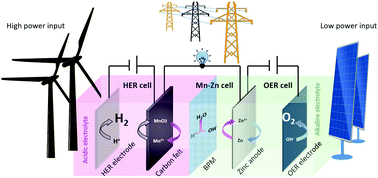
Amphoteric water electrolysis with a bipolar membrane can accommodate optimal pH conditions simultaneously for both cathode and anode under steady-state operation without changing the overall thermodynamics of water splitting. However, the high voltage loss of bipolar membrane imposes significant constraints on operating current density, leading to low current density (<50 mA cm−2) for hydrogen production. In this work, decoupled amphoteric water electrolysis assisted with MnO2/Mn2+ redox mediator demonstrated to separate the stiff couple between hydrogen and oxygen production into two independent processes, which enables hydrogen production to run under high power input (up to 1 A cm−2) with oxygen production under low power input. Furthermore, such an amphoteric decoupled water electrolysis system can be integrated with an Mn–Zn battery, which is able to realize flexible conversion from renewables to hydrogen and electric energy, thus making full use of renewables.


 Please wait while we load your content...
Please wait while we load your content...