Novel anti-Kasha fluorophores exhibiting dual emission with thermally activated delayed fluorescence through detouring triplet manifolds†
Abstract
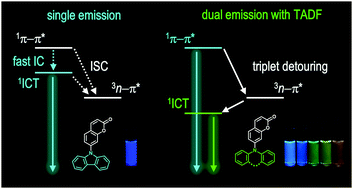
Molecules displaying dual emission (DE) disobey Kasha's rule. Although such molecules are valuable in various applications, the limited availability of DE materials has retarded full utilization of their photofunction. We discovered DE behaviors from a novel series of molecular dyads composed of coumarin and cyclic donors. Our DE dyads are unique in that they display short-wavelength prompt fluorescence and long-wavelength delayed fluorescence. Electrochemical, spectroscopic, quantum chemical, and structural investigations were conducted to elucidate the mechanism underlying the DE behaviors. Our mechanistic studies reveal that DE originates from the high-lying singlet π–π* and the low-lying singlet intramolecular charge-transfer (1ICT) transition states. The two states are under kinetic control between (1) internal conversion and (2) El Sayed-rule-allowed intersystem crossing, followed by reverse intersystem crossing. The latter process enables population of the 1ICT state through detouring via triplet manifolds. In addition, our investigation indicates that DE is also governed by conformeric heterogeneity of the cyclic amino donor between pseudo-axial and pseudo-equatorial forms. These findings are important because they point to an intimate coupling between the photophysical processes of DE and thermally activated delayed fluorescence. Finally, photonic utility of the DE behaviors has been demonstrated by luminescence ratiometric monitoring of the temperature and a triplet quencher.
- This article is part of the themed collection: Journal of Materials Chemistry C Lunar New Year collection 2022


 Please wait while we load your content...
Please wait while we load your content...