11.5% efficient Cu2ZnSn(S,Se)4 solar cell fabricated from DMF molecular solution†
Abstract
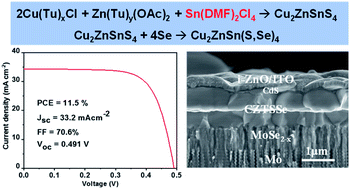
Fabricating a thin film absorber material from a molecular solution is a very promising approach for low-cost and large area photovoltaic technology. The properties of a molecular solution processed kesterite (CZTSSe) absorber highly depends on the components in the solution, which determine the composition of the precursor film and thus the grain growth and properties of the absorber. Here, we report the chemical reactions in N,N-dimethylformamide (DMF) solution using CuCl, Zn(OAc)2, SnCl4, and thiourea (Tu) as precursors and the reaction path from solution to the absorber material. Our results show that SnCl4 reacts with DMF and forms complex Sn(DMF)2Cl4, whereas CuCl and Zn(OAc)2 form complexes with Tu, resulting in an amorphous sulfide kesterite (CZTS) structured precursor film. Monitoring of the selenization process reveals that the precursor film exhibits a direct substitution reaction path and top-down and bottom up bi-directional grain growth, leading to a large grain-composed double layer CZTSSe absorber film with a clean surface. A champion CZTSSe device with an efficiency of 11.5%, an open-circuit voltage of 0.491 V, and a fill factor of 70.6% was achieved. Our results demonstrate that coordination of Sn4+ with a weak ligand (like DMF or DMSO) that can easily de-coordinate during heat-treatment is the key for the formation of a kesterite structured precursor film that can facilitate direct phase transformation grain growth and thus fabrication of high quality kesterite absorber materials.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...