Mechanistic insights into interfaces and nitrogen vacancies in cobalt hydroxide/tungsten nitride catalysts to enhance alkaline hydrogen evolution†
Abstract
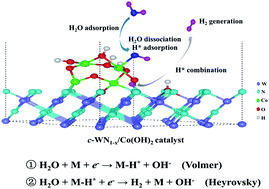
Understanding the limitations of electrocatalytic activity and developing advanced catalysts with enhanced activity are of exceptional importance for future hydrogen energy applications. Due to the complexity of electrolytic hydrogen evolution in alkaline media, there are limited non-noble metal catalysts to afford large scale applications. Tungsten-based catalysts exhibit favorable stability but relatively low activity owing to their strong H* adsorption characteristics. In this work, cobalt hydroxides/cubic phase WN nanoparticles with strengthened interfaces and adjustable nitrogen vacancies anchored on multi-walled carbon nanotubes (Co(OH)2/c-WN1−x/CNTs) are exploited as highly efficient alkaline hydrogen evolution electrocatalysts, which exhibit remarkable activity with an overpotential of 78 mV at 10 mA cm−2 and a low Tafel slope of 43 mV dec−1 in 1 M KOH media. Density-functional theory calculations revealed that the interfacial combinations of Co(OH)2/c-WN1−x were tuned to facilitate the kinetics of hydrogen evolution, where Co(OH)2 promoted the adsorption of water molecules and the H–OH dissociation, and cubic phase WN with nitrogen vacancies (c-WN1−x) accelerated hydrogen generation by optimizing the hydrogen adsorption free energy.


 Please wait while we load your content...
Please wait while we load your content...