Lattice oxygen of PbO2 induces crystal facet dependent electrochemical ozone production†
Abstract
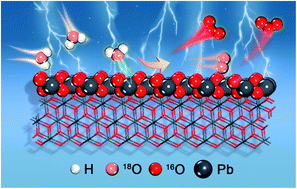
The on-site production of ozone via electrochemical water electrolysis has attracted increasing interest because of its security and efficiency. However, the underlying mechanism of the facet effect and the influence of lattice oxygen on β-PbO2 for electrochemical ozone production (EOP) remain unclear. Here, β-PbO2-120 nanorods (β-PbO2-120 NRs) were prepared to investigate the mechanism of the facet effect and the influence of lattice oxygen during the EOP process. The β-PbO2-120 NRs assembled as an anode in a membrane electrode assembly show a remarkable EOP performance. Measurements using in situ18O isotope-labeling differential electrochemical mass spectrometry confirm that all three oxygen atoms in the ozone originate from the lattice oxygen of β-PbO2. Theoretical calculations verify that the EOP reaction pathway on β-PbO2 follows the lattice oxygen mechanism (LOM), and surface lattice oxygen migration and coupling to O2/O3 are favorable on the (101) and (110) surfaces of β-PbO2. Different reaction mechanisms are proposed on the two surfaces, and (101) exhibits higher reactivity for O2 and the formation of O3. This valuable insight into the facet effect and LOM of metal oxide-based electrocatalysts can be extended to other applications.


 Please wait while we load your content...
Please wait while we load your content...