Phthalimide-based-SSCF3 reagent for enantioselective dithiotrifluoromethylation†
Abstract
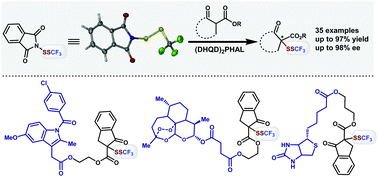
An electrophilic phthalimide-based-SSCF3 reagent was designed and prepared for straightforward SSCF3 incorporation of diverse β-keto esters in natural products, pharmaceuticals and biological molecules. The asymmetric dithiotrifluoromethylation was achieved with a good to excellent enantioselectivity, catalyzed by a cinchona-alkaloid-based catalyst (DHQD)2PHAL via an ammonium-hydrogen-bonded induction mode.


 Please wait while we load your content...
Please wait while we load your content...