Diazadiborinine as an ambiphilic catalyst for metal-free hydrogenation: a computational study on the structural design and reaction mechanism†
Abstract
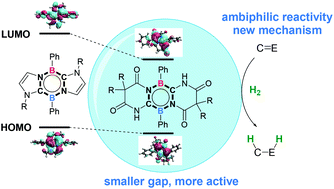
Ambiphilic main group compounds have been emerging as a novel type of metal-free catalyst to activate small molecules. However, the catalytic reactivity of these compounds on hydrogenation is still challenging. In this study, we designed potential ambiphilic compounds by engineering the HOMO and HOMO–LUMO gap of 1,4,2,5-diazadiborinine through modifying the conjugated N-heterocycle carbene moieties. Compound B bearing Bielawski's diaminocarbenes is proposed to be a promising catalyst for the ambiphilic activation of H2. The hydrogenation of typical imines or ketones with H2 by compound B was evaluated using DFT studies. Detailed calculations demonstrated that the hydrogenation of N-methylbenzaldimine and acetophenone takes place through consecutive hydride transfer and protonation processes rather than the conventional concerted hydrogen transfer mechanism. The hydrogenation of N-methylbenzaldimine is initiated by the protonation of the imine nitrogen, followed by the transfer of activated hydride to the iminium carbon. The rate-determining step is the hydride transfer with a free energy barrier of 28.7 kcal mol−1. In contrast, the hydrogenation of acetophenone takes place through the hydride transfer to the carbonyl carbon, followed by the protonation of alkoxide oxygen, via an unprecedented inner-sphere mechanism. The rate-determining step is the hydride transfer with a free energy barrier of 29.9 kcal mol−1. The hydrogenation of N-methylbenzaldimine and acetophenone is exergonic by 9.2 kcal mol−1 and 3.2 kcal mol−1, respectively. These results suggest modified 1,4,2,5-diazadiborinine as a promising ambiphilic catalyst for the hydrogenation of imine or ketone. The newly proposed inner-sphere mechanism via the heterocycle would provide helpful information for the development of ambiphilic main group compounds as metal-free catalysts.


 Please wait while we load your content...
Please wait while we load your content...