Reversible cyclic-linear topological transformation using a long-range rotaxane switch†
Abstract
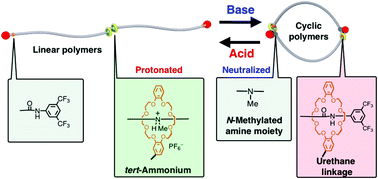
A reversible cyclic-linear topological transformation of a poly(ε-caprolactone) facilitated by a long-range rotaxane switch is reported. A linear polymer, whose topology is fixed by a protonated tert-amine/crown ether interaction at the center of the polymer chain, was reversibly transformed into a cyclic polymer fixed by urethane linkage/crown ether interaction at the polymer end upon neutralization/acidification of the tert-amine moiety.


 Please wait while we load your content...
Please wait while we load your content...
