One-pot synthesis of tetrasubstituted 2-aminofurans via Au(i)-catalyzed cascade reaction of ynamides with propargylic alcohols†
Abstract
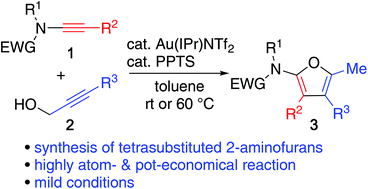
One-pot synthesis of fully substituted 2-aminofurans via a Au(I)-catalyzed cascade reaction of ynamides and propargylic alcohol was realized. Hydroalkoxylation of ynamide with propargylic alcohol, Saucy–Marbet rearrangement, and cyclization of the resultant 3,4-dienamide sequentially proceeds in a one-pot reaction under highly mild conditions to give fully substituted 2-aminofurans.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...