Protecting carbohydrates with ethers, acetals and orthoesters under basic conditions†
Abstract
Chlorinated ethyl and vinyl ethers are introduced at various positions of carbohydrates. Depending on the relative stereochemistry, vinylethers, acetals or orthoesters are formed under basic conditions. The products are stable, but are easily deprotected after dechlorination. The scope of the intramolecular protection is studied using common pentoses and hexoses.
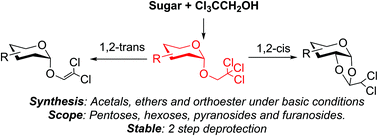
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...