Biological halogen bonds in protein–ligand complexes: a combined QTAIM and NCIPlot study in four representative cases†
Abstract
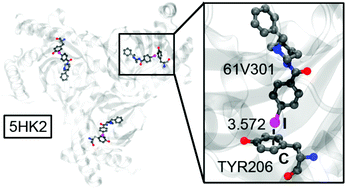
In this study, the PDB has been manually scrutinized by using a subset of all PDB entries containing organic iodinated ligands. Four structures exhibiting short I⋯A halogen bonding (HaB) contacts (A stands for the σ-hole acceptor) have been selected and further analysed. In most hits, the sigma-hole acceptor corresponds to an O-atom of the amido group belonging to the protein backbone. In a minority of hits, the electron donors are O, S, Se or π-systems of the amino-acid side chains. A judicious selection of four PDB structures presenting all four types of HaB interactions (C–I⋯A, A = O, S, Se, π) has been performed. For these selected structures, a comprehensive RI-MP2/def2-TZVP study has been carried out to evaluate the HaB energetically. Moreover, the interactions have been characterized by combining the quantum theory of “atoms-in-molecules” (QTAIM) and the noncovalent interaction plot (NCIPlot) and rationalized using the molecular electrostatic potential (MEP) surface.
- This article is part of the themed collections: Halogen bonding in organic synthesis and catalysis and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...