Asymmetric bis(oxazoline)–Ni(ii) catalyzed α-hydroxylation of cyclic β-keto esters under visible light†
Abstract
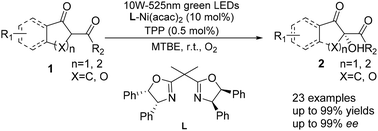
Using visible light as a driving force and molecular oxygen as a green oxidant, we developed bis(oxazoline)–Ni(acac)2 catalyzed asymmetric α-hydroxylation of β-keto esters under low photosensitizer loading, and the protocol enabled an efficient transformation to provide the desired chiral α-hydroxy-β-keto esters in high yields (up to 99%) and enantioselectivities (up to 99% ee) at room temperature.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...