Cyanoalkylation/alkynylation of allylic alcohol through intramolecular radical 1,2-alkynyl migration†
Abstract
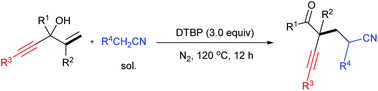
A di-tert-butyl peroxide (DTBP)-promoted difunctionalization of α-aryl α-alkynyl allylic alcohols with alkyl nitriles was developed, affording a series of α-alkynyl γ-cyano functionalized ketones in moderate yields. This procedure involved C(sp3)–H bond cleavage of alkyl nitriles and radical 3-exo-dig cyclization. After this, radical 1,2-alkynyl migration is preferred rather than 1,2-aryl migration.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...