Synthesis and application of a thiol-reactive HBED-type chelator for development of easy-to-produce Ga-radiopharmaceutical kits and imaging probes†
Abstract
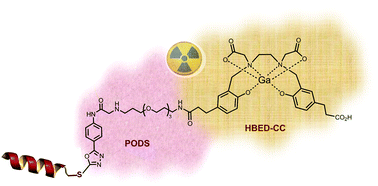
In radiopharmaceutical syntheses, maleimide is commonly used for linking thiol-bearing bioactive molecules to metal–complexing ligands (chelators). However, due to instability of the resulting linkage, phenyloxadiazolyl methylsulfone (PODS) was developed as an alternative to maleimide. This coupling strategy has never been attempted with HBED which is a powerful chelator for gallium-radiolabeling especially at ambient temperature. Here we present HBED-CC-PODS as a bifunctional chelator scaffold for the site-selective conjugation of thiol-bearing vectors and [68Ga]Ga-radiolabeling.


 Please wait while we load your content...
Please wait while we load your content...