Role of substituents in the Hofmann–Löffler–Freytag reaction. A quantum-chemical case study on nicotine synthesis†‡
Abstract
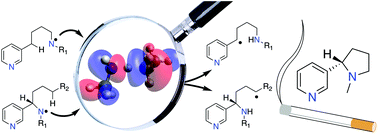
The Hofmann–Löffler–Freytag (HLF) reaction can be successfully used to synthesize saturated heterocyclic nitrogen-containing nature-derived pharmaceuticals such as nicotine and its derivatives. In this study the rate-determining hydrogen atom transfer (HAT) step in nicotine synthesis has been analyzed using quantum chemical methods. Through quantification of substituent effects in the HAT step of the reaction on both nitrogen and carbon atoms, optimized synthetic strategies are outlined for the racemic as well as the stereoselective synthesis of nicotine. This latter process can be achieved using common nitrogen protecting groups, such as Ac, TFAc, and Boc. The said protecting groups show superior nitrogen radical activation as compared to the commonly used Tosyl group. Computational results indicate that the 1,5-HAT step is in this case likely to work even for the reaction with primary unactivated carbon centers.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...