Synthesis of substituted anilines via a gold-catalyzed three-component reaction†
Abstract
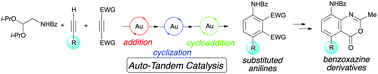
A three-component reaction for the synthesis of substituted anilines by a gold(I)-catalyzed domino reaction was developed. Cationic gold catalysts selectively and sequentially activated two different alkynes, which were involved in pyrrole synthesis and subsequent Diels–Alder reaction. The sequential formal (3 + 2) annulation/Diels–Alder reaction of three components provided a variety of substituted anilines in a modular fashion. Moreover, utility of the aniline products was demonstrated by derivatization to substituted benzoxazines, which are pharmaceutically important heterocycles.
- This article is part of the themed collections: Hybrid Catalysis and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...