Tropane alkaloid biosynthesis: a centennial review
Abstract
Covering: 1917 to 2020
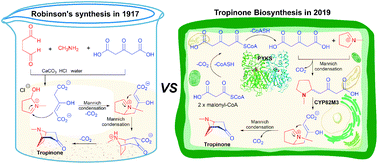
Tropane alkaloids (TAs) are a remarkable class of plant secondary metabolites, which are characterized by an 8-azabicyclo[3.2.1]octane (nortropane) ring. Members of this class, such as hyoscyamine, scopolamine, and cocaine, are well known for their long history as poisons, hallucinogens, and anaesthetic agents. Since the structure of the tropane ring system was first elucidated in 1901, organic chemists and biochemists have been interested in how these mysterious tropane alkaloids are assembled in vitro and in vivo. However, it was only in 2020 that the complete biosynthetic route of hyoscyamine and scopolamine was clarified, and their de novo production in yeast was also achieved. The aim of this review is to present the innovative ideas and results in exploring the story of tropane alkaloid biosynthesis in plants from 1917 to 2020. This review also highlights that Robinson's classic synthesis of tropinone, which is one hundred years old, is biomimetic, and underscores the importance of total synthesis in the study of natural product biosynthesis.


 Please wait while we load your content...
Please wait while we load your content...