Potency and metabolic stability: a molecular hybrid case in the design of novel PF74-like small molecules targeting HIV-1 capsid protein†
Abstract
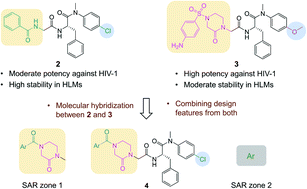
PF74 (1) is a potent and well-characterized prototypical small molecule targeting human immunodeficiency virus type 1 (HIV-1) capsid protein (CA), but not a viable antiviral lead due to the lack of metabolic stability. We report herein our molecular hybridization-based medicinal chemistry efforts toward potent and metabolically stable PF74-like small molecules. The design of the new sub-chemotype 4 rationally combines binding features of two recently reported PF74-like compounds 2 and 3. The subsequent confirmation and structure–activity relationship (SAR) of hit 4a entailed the chemical synthesis of 37 novel analogs, most of which showed modest but meaningful thermal shift, and low μM antiviral activity. The most potent analogs (4a, 4d, 4o, and 4r) all exhibited noticeably improved metabolic stability over PF74. Molecular modeling suggests that these new analogs bind to the PF74 binding site. Overall, our work demonstrated that the molecular hybridization approach is suitable for designing compounds with balanced potency and metabolic stability.
- This article is part of the themed collection: Antibiotic and Antiviral compounds


 Please wait while we load your content...
Please wait while we load your content...